Webinar – The Role of Nimotuzumab in the Treatment of Head and Neck and Nasopharyngeal Cancer

This webinar was conducted on May 28th, 2022 discussing the role of Nimotuzumab in the treatment of locally advanced squamous cell carcinoma of head and neck and nasopharyngeal cancer. The webinar was attended by more than 80 healthcare professional participants from across Asia. There are two speakers, Dr. Kumar Prabhash and Dr. Jiade J. Lu.
– First Topic: A randomized phase III study of Nimotuzumab in Combination with Concurrent RT and Cisplatin versus RT and Cisplatin alone, in LA SCCHN (Dr. Kumar Prabhash)
EGFR overexpression is common in HNSCC and confers poor prognosis. The previous phase II study showed promising results with improvement in outcomes and without any additional increase in adverse events rate compared to control.
This phase III trial primary endpoint was PFS and secondary endpoints were DFS, LRC, OS, and adverse event rate (CTCAE v4.02). The trial design is as follow:
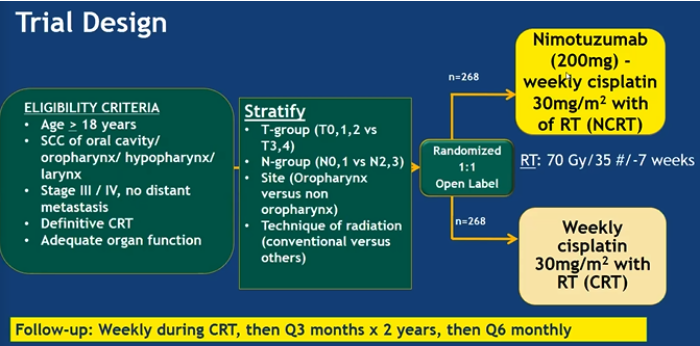
The 2-year PFS was 50.1% in the CRT arm and 61.8% in the NCRT arm (HR 0.69; 95% CI, 0.53-0.89 p = 0.0044). This was clinically and statistically significant. Addition of Nimotuzumab decreased the hazard of disease recurrence by 29% (HR 0.71; 95% CI, 0.55-0.92 p = 0.0074) and locoregional failures by 10% (HR 0.67; 95% CI, 0.50-0.89 p = 0.0059). The OS HR 0.84 (95% CI 0.62-1.13 p 0.16) suggesting a 16% reduction in the risk of death but was not statistically significant.
In conclusion, Nimotuzumab in combination with cisplatin and RT was superior to cisplatin and RT alone in improving the PFS, DFS and LRC. This Nimotuzumab combination provides an alternative therapeutic option in the armamentarium against LA SCCHN especially if weekly cisplatin is planned.
– Second Topic: Phase III Study of Nimotuzumab in Combination with RT following TPF Induction in Locally Advanced NPC, and its Application in Treatment Approach of NPC in China
Radiation therapy is the only curative modality for non-metastatic NPC. Concurrent therapy ± adjuvant is the standard for LA NPC, while induction chemotherapy + concurrent therapy is recommended by NCCN and universally recommended in endemic areas. Recent meta-analysis showed that concurrent radiation (CRT) and CRT + adjuvant chemotherapy (AC) can significantly improve OS in LA NPC. However, the adverse effects from concurrent chemoradiation (CCRT) are significant.
EGFR overexpression is an independent prognostic factor for HNSCC and NPC which is seen in around 90% of those patients. A randomized phase II trial in China was conducted to compare conventional RT vs conventional RT + Nimotuzumab in NPC patients and showed an improved 3-y OS (77.61% vs 84.29%, p<0.05 ) without increasing adverse effects.
This phase III study was conducted to study the toxicity and efficacy of Nimotuzumab vs cisplatin used in concurrent with IMRT. The design is as follow:
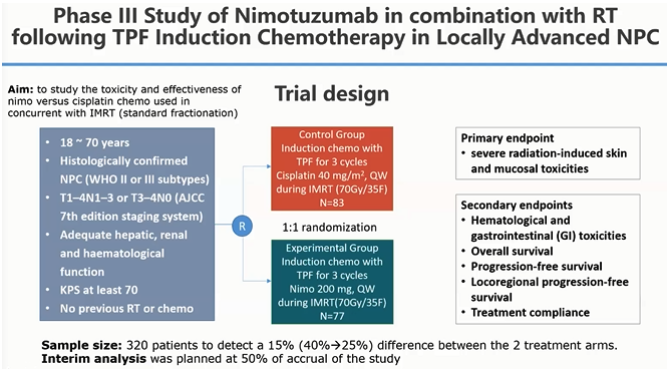
Between the two groups, the response to treatment (CR, PR, SD, PD), OS, PFS, LRPFS, DMFS, all showed similar results or not much difference between IMRT-cisplatin and IMRT-Nimotuzumab. However, the significant difference of toxicity profile between cisplatin and Nimotuzumab was apparent in the concurrent treatment. More patients completed the nimotuzumab concurrent treatment (97.3%) compared to only 40.2% of patients who completed the cisplatin concurrent treatment. Additionally, the adverse effects of neutropenia, thrombocytopenia, anemia, anorexia, nausea/vomiting, and fatigue were all significantly lower in the nimotuzumab group.
In conclusion, the concurrent IMRT with Nimotuzumab or cisplatin following TPF-based chemotherapy produced similar 5-year OS/PFS for LA-NPC. Acute severe dermatitis/mucositis were less in patients receiving Nimotuzumab (28.8% vs 40.2%, p=0.191). Acute severe GI and hematological toxicities were significantly less in patients receiving Nimotuzumab. Moreover, a retrospective study showed that Nimotuzumab has a better safety profile than cetuximab in LA NPC patients receiving CCRT treatment.
